| |
| |
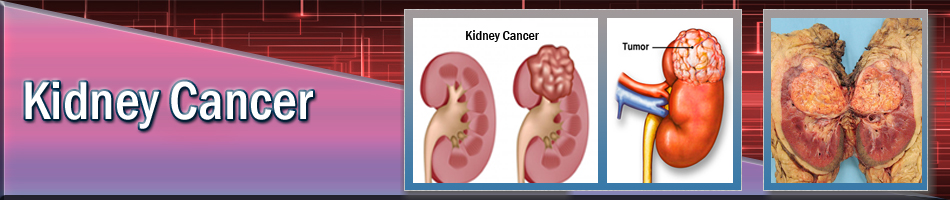
| Kidney Cancer |
|
|
It is important to realize that with timely diagnosis and treatment, kidney cancer can be cured. If found early, the survival rate for patients with kidney cancer ranges from 79 to 100 percent. More than 100,000 survivors of kidney cancer are alive in the United States today. The following information addresses the most common questions about kidney tumors and serves as a supplement to the discussion that you have with your physician. |
| |
|
| What happens under normal conditions? |
|
|
Most people have two functional kidneys. The kidneys produce urine that drains through narrow tubes (called ureters) into the bladder. The kidneys are usually located in each flank protected by muscles of the back and rib cage. The kidneys are contained within a fibrous sheath called the Gerota's fascia and surrounded by a layer of fat. The kidney capsule is a thin layer that covers the outer surface of the kidney (similar to the red peel of an apple). The primary vein that drains the kidney (renal vein) merges with the vein that takes blood to the heart (vena cava). An adrenal gland is located above each kidney within Gerota's fascia.
The adrenal glands, which are not part of the kidney, are located near the top of each kidney. The adrenal glands regulate blood sugar, potassium, body fluids and sex hormones. They also control the body's response to stress by producing a hormone called adrenaline.
The kidney is the main filter of the body and thus performs many bodily functions, such as controlling fluid balance, regulating electrolytes (e.g., sodium, potassium, calcium, magnesium), preventing acid buildup, eliminating waste products, producing urine, and regulating blood pressure. The kidney also manufactures a hormone called erythropoietin that stimulates the production of red blood cells.
When the kidneys are damaged or a significant portion of kidney tissue is removed, the normal processes listed above may be impaired. In most cases, mild to moderate impairment causes very minor problems. In cases when kidney function is severely impaired, dialysis may be required. |
| |
|
| What is a kidney tumor? |
|
|
A kidney tumor is an abnormal growth within the kidney. The terms "mass," "lesion" and "tumor" are often used interchangeably. Tumors may be benign (non-cancerous) or malignant (cancerous). The most common kidney lesion is a fluid-filled area called a cyst. Simple cysts are benign and have a typical appearance on imaging studies. They do not progress to cancer and usually require no follow-up or treatment. Solid kidney tumors can be benign, but are cancerous more than 80 percent of the time.
|
| |
|
| What are some facts about kidney cancer? |
|
|
In the United States, 2 percent of all cancers arise from the kidney. In 2013, it is estimated that 65,150 people will be diagnosed of kidney cancer. It is also estimated that 13,680 people will die from kidney cancer. Kidney cancer is slightly more common in males and is usually diagnosed between the ages of 50 and 70 years. The most common kidney cancer is called renal cell carcinoma.
|
| |
|
| What risk factors are associated with kidney cancer? |
|
|
The following associations may increase the risk of developing kidney cancer:
• smoking
• hypertension
• obesity
• family history of kidney cancer
• chronic kidney failure and/or dialysis
• diet with high caloric intake or fried/sauteed meat
• Von Hippel-Lindau syndrome
• tuberous sclerosis |
| |
|
| What are the symptoms for kidney cancer? |
|
|
Many kidney tumors do not produce symptoms, but may be detected incidentally during the evaluation of an unrelated problem or during routine screening for people who are in high-risk categories (e.g. Von Hippel-Lindau disease, tuberous sclerosis). Compression, stretching and invasion of structures near the kidney may cause pain (in the flank, abdomen or back), palpable mass, and blood in the urine (microscopic or grossly visible). If cancer spreads (metastasizes) beyond the kidney, symptoms depend upon the involved organ.
Shortness of breath or coughing up blood may occur when cancer is in the lung, bone pain or fracture may occur when cancer is in the bone and neurologic symptoms may occur when cancer is in the brain. In some cases, the cancer causes associated clinical or laboratory abnormalities called paraneoplastic syndromes. These syndromes are observed in approximately 20 percent of patients with kidney cancer and can occur in any stage (including cancers confined to the kidney).
Symptoms from paraneoplastic syndromes include weight loss, loss of appetite, fever, sweats and high blood pressure. Laboratory findings include elevated red blood cell sedimentation rate, low blood count (anemia), high calcium level in the blood, abnormal liver function tests, elevated alkaline phosphatase in the blood, and high blood count. In many cases, the paraneoplastic syndrome resolves after the cancer is removed.
|
| |
|
| How is kidney cancer diagnosed? |
|
|
Unfortunately, there are no blood or urine tests that directly detect the presence of kidney tumors.
When a kidney tumor is suspected, a kidney imaging study is obtained. The initial imaging study is usually an ultrasound or CT scan. In some cases, a combination of imaging studies may be required to completely evaluate the tumor. If cancer is suspected, the patient should be evaluated to see if the cancer has spread beyond the kidney (metastasis). An evaluation for metastasis includes an abdominal CT scan or MRI, chest X-ray and blood tests. A bone scan is also recommended if the patient has bone pain, recent bone fractures, or certain abnormalities on their blood tests. Additional tests may be obtained when indicated. Kidney cancer has the tendency to grow into the renal vein and vena cava. The portion of the cancer that extends into these veins is called "tumor thrombus." Imaging studies, particularly CT or MRI, can help determine if tumor thrombus is present.
|
| |
|
| What are the different stages of kidney cancer? |
|
|
The most commonly used staging system for kidney cancer was developed by the American Joint Committee on Cancer (AJCC). The most current version is the 2009 AJCC Staging System. This staging system includes the extent of the primary kidney tumor (T stage), the status of lymph nodes near the kidney (N stage) and the presence or absence of metastases (M stage). In kidney cancer, the lymph nodes near the kidney are referred to as regional lymph nodes. Clinical stage is based on radiographic imaging before surgery, whereas pathologic stage is based on the analysis of surgically removed tissue. Staging the cancer helps predict prognosis and survival. In general, cancers with higher T stage, lymph node metastasis, or distant metastasis have a worse prognosis and shorter survival rates, and these patients need to consider more aggressive treatments.
Grade: Tumor grade is a subjective measure of how aggressive the tumor looks under the microscope; therefore, it is determined from a surgical specimen. Grade cannot be determined from radiographic imaging, blood tests or urine tests. Grade usually ranges from one to three or one to four, with higher numbers indicating a more aggressive tumor. Thus, higher grade implies a worse prognosis.
Stage I: The tumor is confined to the kidney and less than 7.0 cm in size. There is no spread to lymph nodes or distant organs.
Stage II: The tumor is confined to the kidney and greater than 7.0 cm in size. There is no spread to lymph nodes or distant organs.
Stage III: There are several combinations of T and N categories that are included in this stage. These include tumors of any size, with spread into the lymph nodes adjacent to the kidney or into the large veins leading from the kidney to the heart (venous tumor thrombus). This stage does not include tumors that invade into other adjacent organs or those with distant metastasis.
Stage IV: There are several combinations of T, N, and M categories that are included in this stage. This stage includes any cancers that have invaded into adjacent organs such as the colon (large bowel) or the abdominal wall, and those with distant metastases.
Primary tumor (T):
TX: Primary tumor cannot be assessed
T0: No evidence of primary tumor
T1:Tumor 7.0 cm or less, confined to the kidney
T1a:Tumor 4.0 cm or less, confined to the kidney
T1b:Tumor 4.0-7.0 cm, confined to the kidney
T2:Tumor greater than 7.0 cm, limited to kidney
T2a:Tumor> 7 cm and less than 10.0 cm, confined to the kidney
T2b:Tumor> 10 cm, confined to the kidney
T3: Tumor extends into major veins or perinephric tissues but not into the adrenal gland and not beyond Gerota's fascia
T3a:Tumor extends in the renal vein or its segmental branches, or tumor invades perirenal and or renal sinus fat but not beyond Gerota's fascia
T3b:Tumor extends into the vena cava below the diaphragm
T3c: Tumor extends into vena cava above diaphragm or invades the wall of the diaphragm
T4:Tumor invades beyond Gerota's fascia (including contiguous extension into the ipsilateral adrenal gland
N - Regional lymph nodes
NX: Regional nodes cannot be assessed
N0: No regional lymph node metastasis
N1: Metastasis in regional lymph node(s)
N2: Metastasis in more than one regional lymph node
M - Distant metastasis
MX: Distant metastasis cannot be assessed
M0: No distant metastasis
M1: Distant metastasis
|
| |
|
| What are the treatment options for tumors that appear confined to the kidney? |
|
|
When the tumor appears confined to the kidney (a "localized" tumor), there are three main treatment options: tumor removal, tumor ablation and surveillance. Chemotherapy, hormone therapy and radiation therapy are not effective treatments for kidney cancer.
Tumor removal: Tumor removal is considered the standard mode of therapy for most patients and is accomplished by performing a surgery called nephrectomy. Radical nephrectomy is surgical removal of everything within Gerota's fascia, including the whole kidney. Partial nephrectomy is surgical removal of part of the kidney (in this case, the part that contains the tumor). The goal of partial nephrectomy is to remove the entire tumor while preserving as much normal kidney tissue as possible. The kidney tissue that is conserved may prevent the need for dialysis if subsequent kidney damage occurs. Nephrectomy can be performed through a traditional incision (open surgery) or through several small incisions (laparoscopic or retroperitoneoscopic surgery). Partial nephrectomy is now considered the reference standard for the management of confined kidney tumors, because it saves as much kidney function as possible. Loss of kidney function is associated with an increased risk of cardiovascular events and reduced survival bases on several recent studies in the field.
Open nephrectomy (radical and partial): Traditional open nephrectomy (partial or radical) is performed through a flank or abdominal incision. This incision is typically 3-8 inches in length and may include removal of a rib. In the past, open radical nephrectomy was considered the treatment of choice for tumors that appeared to be confined to the kidney. However, five- to 10-year follow up reveals that partial and radical open nephrectomies provide equally effective cancer treatment for many patients with a single, small, localized tumor. Therefore, partial and radical nephrectomies are now considered standard treatments. If you are interested in partial nephrectomy, it is important that you seek a urologist who has experience with this technique.
As stated before, partial nephrectomy is performed to preserve as much normal kidney tissue as possible; however, its complication rate may be slightly higher than radical nephrectomy. Open partial nephrectomy is usually the treatment of choice when radical nephrectomy results in either immediate dialysis or a high risk for subsequent dialysis, such as when the patient has a single functioning kidney, poor overall kidney function, medical or genetic diseases that threaten kidney function or bilateral kidney tumors. Partial nephrectomy is usually not recommended in patients with tumors that have any of the following characteristics: extension into the renal vein, close proximity to the main kidney vessels or factors that would make complete tumor resection unlikely. When the tumor cannot be safely removed by partial nephrectomy, radical nephrectomy is performed. If you elect to undergo a partial nephrectomy, there is always a risk that the entire kidney may need to be removed.
Laparoscopic radical nephrectomy: Laparoscopic nephrectomy is performed using telescopes that are inserted into the abdominal cavity through small "key hole" incisions; however, a somewhat larger incision is often made to permit removal of an intact kidney. Nephrectomy performed by inserting the telescopes into the cavity that surrounds the kidney (rather than into the abdominal cavity) is called retroperitoneoscopic nephrectomy.
Current data indicate that open and laparoscopic radical nephrectomies have similar complication rates and provide equally effective cancer treatment for patients with tumors that appear confined to the kidney. Compared to open radical nephrectomy, laparoscopic radical nephrectomy has less postoperative pain, shorter hospital stay and shorter recovery time. If you elect to undergo a laparoscopic radical nephrectomy, there is a low risk (usually less than five percent) that the surgeon will need to convert to an open nephrectomy (i.e., convert the "key hole" incisions to a larger incision). Not all patients are candidates for laparoscopic nephrectomy. Laparoscopic radical nephrectomy is best suited for small, localized tumors that have not invaded the lymph nodes or renal vein. Open nephrectomy is preferred in patients with severe scarring around the kidney or a history of extensive abdominal surgery. Surgeons who are experienced with retroperitoneoscopy may consider this approach in patients with a history of extensive abdominal surgery.
Laparoscopic and retroperitoneoscopic partial nephrectomy:
Information is accumulating on laparoscopic (or robotic) and retroperitoneoscopic partial nephrectomy, and these are good approaches in many patients. In general, this approach is best suited for relatively small, peripherally located tumors that are relatively easy to remove and for which reconstruction of the kidney is straightforward.
Tumor ablation:Tumor ablation destroys the tumor without surgically removing it. Examples of ablative technologies include cryotherapy, interstitial radiofrequency ablation, high- intensity focused ultrasound, microwave thermotherapy and laser coagulation. Ablation can be accomplished during open surgery, laparoscopy, retroperitoneoscopy or percutaneously (through the skin). Since renal tumor ablation is a relatively new procedure, long-term results are not as well defined, and tumor recurrences appear to be somewhat more common than after surgical excision. However, ablation may be less invasive than nephrectomy and may be useful in patients who cannot tolerate a more extensive surgery. Tumor ablation may also permit a better chance of preserving kidney function in situations when multiple tumors are present. In general, tumor ablation is best reserved for older or somewhat frail patients.
Embolization:
This is not a standard treatment option, but may be considered in patients who cannot tolerate tumor removal or ablation. It may also be considered as an adjunct to standard forms of treatment, especially when the tumor is actively bleeding. Embolization can stop the bleeding and permits physicians to stabilize the patient before surgery. Embolization is usually performed under sedation and is accomplished by advancing a long narrow catheter from a peripheral artery (such as in the groin) into the artery of the kidney. The catheter is used to deposit small embolic material particles in the vessels of the kidney. These particles block the flow of blood to the tumor and, therefore, stop active bleeding. Furthermore, without a blood supply, the tumor eventually dies. Since it is unclear whether or not embolization completely eliminates the tumor, it is not considered a primary form of therapy for kidney cancer. Embolization will not kill the tumor, but rather will just stop or slow down the bleeding. It is thus best viewed as a palliative procedure or an adjunctive procedure when used in combination with surgery. |
| |
|
| What are the treatment options for tumors that invade the renal vein or vena cava? |
|
|
When tumor invades into the renal vein or vena cava, open surgery is recommended to remove the affected kidney and to extract the tumor from the veins. It is important that you seek a urologist who has experience with this type of surgery. This is a major operation that requires isolation and clamping of the inferior vena cava, the largest vein in the body. After the blood flow is blocked the vein is opened and the tumor thrombus is extracted. The vein is then sutured closed. Sometimes embolization is performed before tumor removal. Embolization may also be considered in patients who cannot tolerate surgery.
|
| |
|
| What are the treatment options for tumors that have spread to other organs? |
|
|
When the tumor has spread to other organs, there have traditionally been four primary treatment options: nephrectomy followed by immunotherapy, initial treatment with immunotherapy, clinical research trials and surveillance. More recently, a new category of treatment has been added, namely treatment with drugs that block the blood flow into the cancer (targeted agents, see below).
Immunotherapy: Immunotherapy stimulates your immune system to attack cancer. Hopefully, the immune system will eliminate cancer in much the same way it eliminates the flu. The most commonly used immunotherapy agents are interleukin-2 (IL-2) and interferon. Until recently, IL-2 was the only effective therapy approved by the Food and Drug Administration (FDA) for the treatment of metastatic kidney cancer. Approximately 20 percent of patients respond to immunotherapy with some degree of tumor regression. Approximately 5-7 percent of patients have complete cancer regression—most of those patients have been treated with the high dose IL-2 protocol. Many different immunotherapy regimens have been studied. One of the most effective regimens is high dose bolus IL-2, which requires inpatient hospitalization. During the initial hospitalization, intravenous IL-2 is administered over five days. The patient is usually allowed to go home for a rest period of five to10 days. Then, the patient is readmitted to the hospital for another five-day course of intravenous IL-2. The most common side effects of immunotherapy are similar to flu symptoms and include fever, chills, nausea, vomiting, diarrhea and fatigue. Other side effects include low blood pressure, fluid accumulation in the lungs (pulmonary edema), impaired liver function, impaired kidney function, mental status changes (such as confusion, agitation, disrupted sleep pattern), rapid heartbeat and irregular heartbeat. Most side effects are temporary and subside when the immunotherapy is stopped. To be a candidate for immunotherapy, the patient must be in good general condition, have adequate function of vital organs (such as the heart, lungs and kidneys) and have no brain metastasis. Immunotherapy is not effective against cancer in the brain. Before immunotherapy, patients must have tests to assess vital organ function and a scan to determine if brain metastases are present.
Nephrectomy followed by immunotherapy or antiangiogenic therapies: In patients with metastases, the best chance of survival is achieved by removing the affected kidney before administering immunotherapy or targeted agents. The kidney may be removed by open or laparoscopic surgery. This treatment option is only offered when the patient is a candidate for both nephrectomy and systemic therapy. Therefore, patients should not undergo any treatment until they have been evaluated by both an oncologist who specializes in immunotherapy and a urologic surgeon. Treatment should only be initiated after the surgeon and oncologist agree that the patient is a candidate for both nephrectomy and systemic therapy. Patients must be relatively healthy and the burden of disease must be somewhat limited.
Initial treatment with immunotherapy: In some patients, surgery may be too risky. These patients may be treated initially with systemic therapy. If they respond adequately and if their medical condition improves, they may undergo surgical removal of the remaining tumor.
Targeted Agents:Tumors must stimulate the ingrowth of blood vessels to provide them with nutrients and oxygen. This process, also known as angiogenesis, is essential for a tumor to continue to grow and to metastasize to other areas of the body. Kidney cancers are very angiogenic and are known to be some of the most vascular tumors in the body. They do this by secreting a protein called vascular endothelial growth factor, or VEGF. VEGF acts on nearby blood vessels stimulating them to sprout new vessels to supply the tumor. Recently new drugs have been developed that block the action of VEGF, and thereby cause the vessels supplying the tumor to regress. This starves the tumor and slows it down. Other agents block the mTOR protein in the cell that drives cancer growth. All of these drugs are known as anti-angiogenic or targeted treatments. Several targeted agents are now approved by the FDA for the treatment of patients with advanced kidney cancer including sorafenib (Nexavar), sunitinib (Sutent), pazopanib, bevacizumab, temsirolimus and everolimus. Recent studies show that these drugs can slow the progress of kidney cancer and allow patients to live longer. While a complete cure is still an uncommon event, this is still a step forward. These drugs are taken orally or intravenously, but they can be associated with side effects including fatigue, hypertension, and skin rashes. But most patients are able to tolerate these drugs fairly well and appear to benefit from them.
Clinical research trials: Research protocols are not available to all patients. If you are interested in finding out more about these protocols, ask your doctor, check with your local academic institution or search the Internet. There are many non-standard therapies that are being studied in research trials. Some of these therapies include cellular immunotherapy, tumor vaccines, gene therapy, stem cell transplants, anti-angiogenesis therapy, inhibitors of growth factors, etc. Although these therapies appear promising, they are still experimental and it is unclear whether or not they are effective treatments for kidney cancer.
Radiation: Radiation therapy is not used to cure kidney cancer, but rather for alleviation of symptomatic metastasis. For example, the pain from bone metastases can be relieved by radiation to bone lesions. It may be used alone or in combination with other therapies.
Surveillance: May be appropriate when any of the following are present: the kidney tumor has a low probability of being cancer; the patient cannot tolerate treatment; the patient has a short life expectancy (i.e., they are likely to pass away from other causes); or the patient does not want treatment. With lesions that have a low probability of being cancer, regular follow up with a physician is mandatory. Angiomyolipoma, a benign tumor, is the only kidney tumor that can be diagnosed by CT scan. Patients with angiomyolipoma may undergo surveillance with periodic imaging studies. However, embolization or surgical removal (preferably by partial nephrectomy) may be necessary when the angiomyolipoma is symptomatic, bleeding or greater than four centimeters in size.
|
| |
|
| What can I expect after treatment for kidney cancer? |
|
|
After treatment for kidney cancer, routine life-long surveillance is necessary. Surveillance typically consists of periodic assessment by a physician, blood tests and X-rays. There is no standard surveillance protocol; therefore, your physician will determine the necessary tests and their timing based on your unique situation. In general, tumors of advanced stage are higher risk and require more intensive surveillance.
Kidney function: When kidney tissue is removed (nephrectomy) or destroyed (by ablation or embolization), the remaining functional kidney tissue usually works sufficiently to avoid problems. Nonetheless, kidney function should be assessed periodically after treatment. In a patient who undergoes a radical or partial nephrectomy on one side and has a normal kidney on the other side, the need for dialysis is extremely rare. In fact, people can live a normal life with only one functioning kidney. When a patient has only one kidney (a "solitary" kidney) and part of that kidney needs to be removed, the risk of permanent dialysis is 4 to 7 percent, the risk of temporary dialysis is 3.5 percent
and the risk of impaired kidney function that does not require dialysis is 26 to 33 percent. In most cases, kidney dysfunction is temporary and improves without treatment. When the remaining functional renal tissue is less than an entire kidney, there is a risk that the function of that tissue will deteriorate over time. This deterioration is called "hyperfiltration renal injury" and may occur up to 10 years after surgery. In some cases, hyperfiltration renal injury can be prevented through careful monitoring. Therefore, in patients with less than one whole kidney remaining, close surveillance of kidney function is particularly important and should include blood pressure measurement and 24-hour urine collection to carefully assess remaining kidney function.
Prognosis: There are many factors that affect outcome after treatment for kidney cancer. However, the two most important prognostic factors are tumor stage and grade. The basic concept is that the more extensive the tumor (and thus the higher the stage), the less likely that treatment will achieve cure. Higher grade also implies a lower chance of cure. Since there are limited long-term data for tumor ablation, the cure rates for these techniques are not as well established. The chance of cure with long-term follow-up is well defined for partial and radical open nephrectomy. In addition, long-term follow up for laparoscopic radical nephrectomy has been accumulating and appears to be similar to open radical nephrectomy, assuming careful patient selection (some patients are not good candidates for laparoscopic surgery and should have conventional surgery).
|
| |
|
| Frequently asked questions: |
| |
|
Should I have a biopsy of the tumor? |
|
|
For kidney tumors, biopsy is usually not indicated because more than 80 percent of solid kidney tumors are cancer and biopsies have a risk of inaccurate results. A false-negative means that the biopsy shows no cancer when cancer is actually present. In other words, the biopsy missed the cancer. A false-negative may occur because the tissue obtained was not adequate to determine a diagnosis, the needle missed the lesion (and hit normal surrounding tissue) or the needle hit the lesion but did not hit the cancer. The latter reason can be explained by the fact that some benign tumors can coexist with cancer. For example, oncocytoma (a benign tumor) may coexist with kidney cancer in up to 10 percent of cases. Thus, a biopsy may sample the benign component, but miss the malignant component. The exact rate of false-negatives is unknown, but studies suggest that they occur six to15 percent of the time. Since a biopsy may miss cancer, the safest approach is to remove the tumor even if the biopsy is negative. In cases where kidney cancer is likely, the current standard of practice is to remove the tumor without performing a biopsy. In very select situations, a biopsy may be recommended when the clinical scenario and radiographic images suggest that the lesion may be from infection, inflammation or cancer from another organ.
|
| |
|
| Do I need a lymph node dissection? |
|
|
Lymph nodes are part of the immune system and are located throughout the body. Channels (called lymphatics) absorb excess fluid from the tissue and transport it through the lymph nodes. Lymph nodes are connected by the lymphatics to form node chains. As the fluid flows down the chain, the lymph nodes “filter out” contaminant. After the fluid completes its journey through the node chain, it flows into the blood stream. Cancer cells that float into the lymphatics can be trapped in lymph nodes and grow into satellite cancers. Over time, these satellite cancers can spread to other lymph nodes in the chain. The presence of enlarged lymph nodes, which is called lymphadenopathy, suggests that these nodes may contain cancer. A lymph node dissection is surgical removal of the regional lymph nodes. Some cancers tend to spread primarily through the lymphatics in a well-defined pattern. In kidney cancer, the pattern of spread through the node chain is quite variable, making it difficult to determine which lymph nodes need to be surgically removed. Furthermore, kidney cancer spreads through the lymphatics and blood with equal frequency. Thus, the cancer may spread directly through the blood stream without reaching the lymph nodes. In patients without lymphadenopathy, lymph node dissection has not been shown to improve the survival. For these reasons, the routine use of a formal lymph node dissection is controversial. For tumors that are localized without enlarged lymph nodes and at low risk for metastasis (clinical stage T1 or T2, N0 M0), formal lymph node dissection is rarely beneficial. In patients with enlarged regional lymph nodes and distant metastasis (clinical stage N1 M1 or N2M1), recent data suggest that lymph node dissection improves survival. In summary, lymph node dissection is usually unnecessary in patients with localized tumors and normal size lymph nodes. However, regional lymph node dissection should be performed in patients with metastasis and enlarged lymph nodes.
|
| |
|
| Does the adrenal gland need to be removed along with the kidney? |
|
|
During a traditional radical nephrectomy, everything within Gerota's fascia is removed, including the adrenal gland. However, recent studies reveal that clinical stage T1 or T2 tumors located in the middle or lower part of the kidney (away from the adrenal) rarely involve the adrenal gland. If two adrenal glands are present, the one adjacent to the involved kidney should be removed when any of the following criteria are present: the adrenal gland appears to contain tumor based on CT scan or intraoperative findings, the tumor is near the adrenal gland (in the upper portion of the kidney) or the tumor is clinical stage T3 or T4. When the patient has bilateral kidney tumors or a solitary adrenal gland, sparing the adrenal gland may be considered even when the previous criteria are present. When the tumor is clinical stage T1 or T2 and located in the middle or lower part of the kidney, leaving the adrenal behind is associated with minimal risk and may prevent future problems.
A person can live a normal life with only one adrenal gland. When both adrenal glands are removed, a person can survive; however, simulating the function of the adrenal glands with medication can be problematic. The goal of sparing the adrenal gland is to preserve as much normal adrenal tissue as possible. The adrenal tissue that is conserved may prevent subsequent problems if the opposite adrenal gland needs to be removed.
|
| |
|
| Do I need any additional treatment after surgery? |
|
|
When the tumor is benign, no further therapy is needed. When the tumor is malignant, most cases can be adequately treated with surgery alone. Additional treatment (in the form of immunotherapy or anti-angiogenic treatments) may be considered when there is advanced local cancer stage (T3 or T4), spread to the lymph nodes or metastasis to distant organs.
Can I live a normal life with one kidney?
Most patients can live a normal life with a single, adequately functioning kidney. Even in cases when the remaining kidney is functioning sub-optimally, the patient may still be able to live a normal life. Dialysis is rarely necessary.
After kidney surgery, can I do anything to protect my remaining kidney?
In patients with only one kidney, it may be prudent to avoid collision/contact sports (football, hockey, boxing, soccer, basketball, etc.) and limited contact sports (baseball, gymnastics, skiing bicycling, etc.) to prevent traumatic injury to the remaining kidney. It may also be prudent to avoid routine use of medicines that can cause kidney damage. Medicines such as non-steroidal anti-inflammatory drugs (ibuprofen, aspirin, etc.) and intravenous iodine contrast (primarily used for certain X-rays) can cause kidney damage in rare situations. Thus, it is best to use these substances only when necessary.
Some medical conditions, such as high blood pressure (hypertension), diabetes, high cholesterol and obesity, have the potential to cause deterioration of kidney function. Treatment of these conditions may prevent kidney damage. Therefore, it is important that you comply with physician-supervised treatment of these conditions. Routine follow up of kidney function is recommended. If your physician detects evidence of hyperfiltration renal injury, additional preventative therapy may be instituted.
|
| |
|
| What can I do to prevent the recurrence of kidney cancer? |
|
|
There are no proven ways to prevent recurrence of kidney cancer; however, it may be prudent to stop smoking. It is extremely important to follow up with your physician on a regular basis.
|
| |
|
| |
|
|